Coding Regions of Intrinsic Disorder Accommodate Parallel Functions: A Dance of Structure and Information
Within the intricate ballet of life, proteins play a starring role, their movements by the genetic code. But proteins are not solely actors; their sequences can hold multiple scripts, allowing them to perform parallel functions like virtuoso performers juggling melodies. And the stage for this remarkable dual act often lies within regions of intrinsic disorder (IDRs).
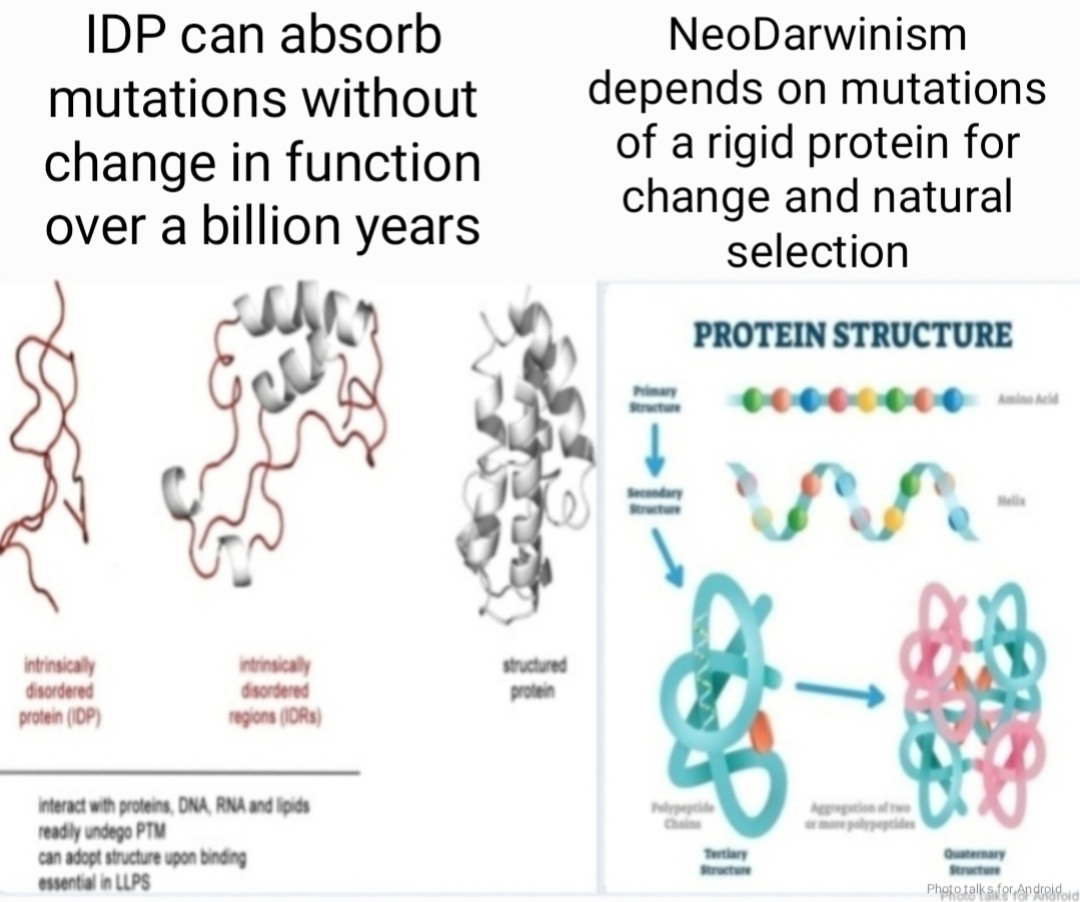
Traditionally, proteins were viewed as rigid, well-defined structures whose every fold and turn dictated their function. IDRs, however, challenge this paradigm. Lacking strict three-dimensional shapes, they resemble flexible dancers, swaying to the music of interacting molecules. This very flexibility, once considered a handicap, is now recognized as a superpower, enabling IDRs to accommodate diverse functions within their coding sequences.
The Unstructured Advantage:
The absence of a fixed structure in IDRs allows them to tolerate mutations more readily than their structurally-constrained counterparts. This tolerance opens the door for the emergence of new functions within the coding sequence without compromising the original one. Imagine a protein like a tapestry - weaving in a new function is easier if the fabric is loose and flowing, as opposed to a tightly woven canvas.
This permissiveness fosters the coexistence of protein-level functions, like binding to other molecules, alongside nucleotide-level functions like regulating gene expression. IDRs can act as platforms for protein interactions, providing binding sites for diverse partners without disrupting their own internal structure. Moreover, the disordered nature of IDRs makes them adept at interacting with DNA and RNA, influencing their transcription and translation.
A Case in Point:
Consider the intrinsically disordered protein p53, a central player in cancer suppression. While its primary function is to activate genes that halt cell division in response to DNA damage, p53 also harbors hidden talents. Its coding sequence contains elements that can influence RNA processing, regulate its own translation, and even interact with non-coding RNAs. This repertoire of parallel functions, enabled by the flexible nature of IDRs, demonstrates the versatility of these enigmatic protein regions.
Evolutionary Implications:
The ability of IDRs to accommodate multiple functions within their coding sequences has profound evolutionary implications. It may explain the rapid emergence of new functions in complex organisms, as novel interactions and regulatory roles can arise within existing IDRs without disrupting their established duties. This "tinkering" with existing structures, facilitated by the tolerance of IDRs, could be a driving force in species diversification and adaptation.
However, this flexibility comes with a cost. The lack of defined structure makes IDRs more susceptible to aggregation and misfolding, potentially leading to disease states. Understanding the delicate balance between functional versatility and potential harm is crucial for deciphering the role of IDRs in health and disease.
Unraveling the Code:
As we delve deeper into the world of IDRs, the question arises: how do these regions manage to juggle multiple functions without getting tangled in the process? Researchers are now exploring the intricate language encoded within the sequences of IDRs. Specific amino acid motifs and post-translational modifications may act as punctuation marks or stage directions, dictating how IDRs fold and interact with other molecules. Deciphering this code will be key to unlocking the full potential of these fascinating protein regions.
The coding regions of IDRs are not simply passive sequences; they are bustling marketplaces where protein and nucleotide-level functions coexist and interact. This remarkable versatility, driven by the intrinsic flexibility of IDRs, opens a new chapter in our understanding of protein function and evolution. As research continues, we may witness the emergence of innovative therapeutic strategies that target IDRs, harnessing their dual nature to combat diseases and advance human health. In the grand theatre of life, IDRs remain a captivating act, their flexible dance holding the potential to rewrite the script of biological complexity.
Intrinsic Chaos: How Disordered Proteins Challenge the Modern Synthesis
The textbook picture of biology paints a neat dichotomy: genes code for proteins, proteins fold into defined structures, and structures dictate function. But nature, ever the rule breaker, throws in a curveball with intrinsically disordered proteins (IDPs). These enigmatic molecules lack stable, three-dimensional shapes, existing instead as floppy, dynamic chains. And here's the kicker: within their seemingly unstructured chaos, IDPs harbor a surprising secret – the ability to execute multiple functions simultaneously. This challenges the very heart of the Modern Synthesis, the dominant framework for understanding life, raising exciting questions about protein evolution and cellular complexity.
The Modern Synthesis, forged in the 20th century, elegantly linked Darwinian evolution with Mendelian genetics. It portrayed genes as blueprints for proteins, their sequences dictating a single, well-defined structure that underpins their function (sequence hypothesis). But IDPs stand in stark contrast to this rigid paradigm. Their flexible coils can interact with multiple partners simultaneously, like a chameleon adapting to its surroundings. This dynamic nature allows them to juggle diverse tasks, acting as molecular switches, scaffolds, and chaperones all at once. Imagine a single protein acting as a translator, a traffic cop, and a bouncer within the bustling cell – that's the versatility of IDPs.
This functional multitasking throws a wrench into the one-gene-one-function assumption of the Modern Synthesis. How can a single sequence encode for such diverse activities? The answer lies in the very lack of structure. The absence of a rigid framework frees IDPs from the constraints of traditional structure-function relationships. Instead, their function emerges from the dynamic interplay of their flexible sequences with different cellular partners. Think of it as a jazz ensemble, where individual notes alone hold little meaning, but their interplay within the improvisation creates a vibrant melody.
Furthermore, the inherent flexibility of IDPs makes them surprisingly tolerant of mutations. Changes that would be detrimental to a rigidly structured protein often go unnoticed in an IDP, allowing them to evolve new functions more readily. This rapid adaptability could explain the prevalence of IDPs in complex organisms, where efficient multitasking and rapid evolution are crucial for survival.
The discovery of IDPs challenges us to rewrite the textbook on protein function. They are not static machines, but dynamic dancers, weaving a intricate tapestry of cellular processes. Their ability to accommodate parallel functions suggests an alternative view of evolution, where gene sequences encode not just structures, but a repertoire of possible interactions and functions waiting to be unleashed. Understanding how IDPs work offers exciting possibilities for biotechnology and medicine, from designing novel drugs that mimic their flexibility to manipulating their interactions for therapeutic purposes.
In conclusion, IDPs are not the protein world's outcasts, but rather its hidden masters of versatility. Their intrinsic disorder challenges the paradigm of the Modern Synthesis, opening doors to a richer understanding of protein evolution and cellular complexity. As we unravel the secrets of these molecular chameleons, we get a glimpse into a cellular world far more dynamic and fascinating than we ever imagined.
Source Article & Snippets
Coding Regions of Intrinsic Disorder Accommodate Parallel Functions
The degeneracy of the genetic code enables multiple meaningful codes to exist in parallel
Eukaryotic proteins are rich in intrinsically disordered proteins/regions (IDPs/IDRs) that mainly fulfill regulatory roles by means of short linear protein interaction motifs.
Lacking structural constraints, IDPs/IDRs are more tolerant to mutations than structured domains.
A range of recent observations implies that the coding regions of IDPs/IDRs are more prone to accommodating parallel codes.
Recently acquired coding regions tend to encode IDPs/IDRs and often develop overlapping functions.
Accumulating evidence suggests that such additional, overlapping functions occur preferentially in the coding sequences of intrinsically disordered proteins/regions (IDPs/IDRs), especially in those that are newly incorporated and thus have reduced selective pressure
It is the lack of strict structural constraints that makes disordered proteins more tolerant to mutations and thus more permissive to the appearance of overlapping functions within their coding sequences than structured domains.



Comments
Post a Comment