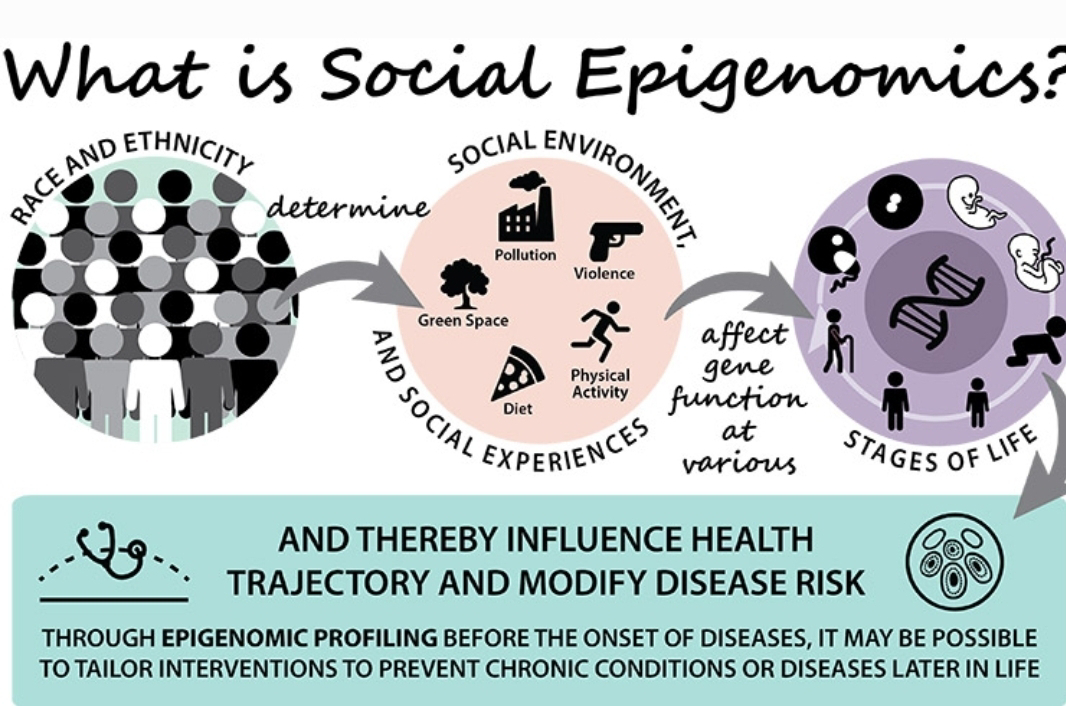
Epigenomics and Gene Regulation in Mammalian Social Systems: A Dance Between Nature and Nurture
The intricate tapestry of social behavior in mammals is woven from a complex interplay between genes and environment. While DNA dictates the blueprint of an organism, recent advancements have revealed a fascinating layer of regulation – epigenetics. This field explores how the social environment can leave its mark on the epigenome, influencing gene expression and shaping behavior across generations. This essay delves into the burgeoning realm of social epigenomics, exploring how social experiences sculpt the epigenome and how these changes, in turn, modulate social behaviors in mammals.
The Epigenetic Landscape: Beyond the DNA Sequence
DNA, the hereditary material, carries the instructions for building and maintaining an organism. However, these instructions alone don't dictate an organism's complete story. Epigenetics adds another dimension, influencing how genes are expressed without altering the underlying DNA sequence. This layer of control is achieved through chemical modifications like DNA methylation and histone acetylation. Imagine DNA as a musical score, while epigenetics acts like the conductor, determining which instruments play and how loudly.
Social Epigenomics: The Social Environment as a Sculptor of Epigenomes
Social epigenomics unveils a remarkable phenomenon: the social environment can influence the epigenome. Social experiences such as dominance hierarchies, parental care, and social isolation can leave epigenetic marks that alter gene expression. For instance, studies in mice have shown that low-ranking individuals in a social hierarchy exhibit increased DNA methylation in genes related to stress response.
This suggests that the chronic stress of subordinate status leaves an epigenetic signature, potentially influencing the individual's ability to cope with future challenges.
Mechanisms of Social Epigenomic Regulation
The intricate dance between social experiences and the epigenome involves several key players.
Stress hormones: Social stressors like aggression or competition can trigger the release of stress hormones like cortisol. These hormones can interact with enzymes that modify DNA methylation patterns, leading to long-lasting epigenetic changes.
Non-coding RNAs: These RNA molecules can act as epigenetic regulators, influencing chromatin structure and gene accessibility. Social experiences can alter the expression of these non-coding RNAs, impacting gene regulation.
Early life experiences: The early social environment, particularly parental care, plays a crucial role in shaping the epigenome. Maternal licking and grooming in rodents, for example, have been linked to epigenetic modifications that promote stress resilience in offspring.
These mechanisms highlight the dynamic nature of the epigenome, constantly adapting to the social landscape.
Consequences of Social Epigenetic Regulation: Shaping Social Behavior
Epigenetic modifications triggered by social experiences can have profound consequences for social behavior.
Social hierarchy: Epigenetic changes associated with dominance or subordination can influence aggression, anxiety, and social interactions, potentially reinforcing the existing social structure.
Reproductive behavior: Studies suggest that social cues can influence epigenetic marks on genes related to reproduction. This could explain how social factors can impact fertility or parental behavior.
Social recognition: Epigenetic regulation might play a role in social memory, allowing individuals to recognize and respond appropriately to familiar social cues.
These examples demonstrate how social epigenomics plays a crucial role in shaping the social lives of mammals.
Intergenerational Effects: The Legacy of Social Experiences
A particularly intriguing aspect of social epigenomics is the potential for transgenerational inheritance. Social experiences of parents can leave epigenetic marks on their sperm or egg cells, potentially influencing the social behavior of their offspring. For example, studies in mice have shown that offspring of mothers exposed to chronic stress exhibit altered stress responses themselves, suggesting a social environment-mediated inheritance of behavior through epigenetic modifications. This raises fascinating questions about the long-term consequences of social experiences and the potential for social environments to shape the trajectory of populations across generations.
The Future of Social Epigenomics: Unveiling the Complexities
Social epigenomics is a rapidly evolving field with immense potential to revolutionize our understanding of social behavior. Future research will delve deeper into:
Identifying specific epigenetic marks: Pinpointing the precise epigenetic modifications associated with specific social experiences will be crucial for understanding the underlying mechanisms.
Long-term consequences: Investigating the long-term effects of social epigenomic changes on behavior across generations will shed light on the social environment's lasting impact.
Therapeutic interventions: Exploring the possibility of manipulating the epigenome to address social disorders like anxiety or aggression could pave the way for novel therapeutic strategies.
The field of social epigenomics unveils a captivating interplay between nature and nurture. By revealing how social experiences sculpt the epigenome, we gain a deeper understanding of the intricate dance between genes and environment in shaping mammalian social behavior. This knowledge has the potential not only to enrich our understanding of social systems but also to unlock novel avenues for promoting social well-being and potentially
The Social Epigenome: Rewriting the Rules in Mammals
The modern synthesis (MS), of evolutionary biology, emphasizes the role of DNA sequence and natural selection in shaping traits. However, a new frontier - social epigenomics - challenges this view by revealing how social interactions can modify gene expression in mammals, potentially influencing behavior and even transmitting these changes across generations.
Social epigenomics explores how the intricate dance of social life leaves its mark on the epigenome, the layer of chemical instructions that regulates how DNA is read. Imagine DNA as the sheet music, and the epigenome as the conductor who determines which instruments play and how loudly. Social experiences like competition for dominance or parental care can trigger epigenetic modifications, such as attaching methyl groups to DNA or altering histone proteins that package it. These modifications don't change the DNA code itself as with the MS but they can influence which genes are turned on or off, shaping an individual's physiology and behavior.
For example, in naked mole rats, social status dictates who reproduces. Studies show that low-ranking females have different epigenetic patterns compared to dominant breeders, potentially affecting genes related to fertility. Similarly, social stress in mice can lead to epigenetic changes in the offspring, impacting their stress response and social behavior.
These findings challenge the modern synthesis by suggesting that social experiences can have a transgenerational effect, potentially influencing the development and behavior of future generations. Evolution can now be seen as shaped not just by DNA mutations, but also by how the environment interacts with the epigenome.
Social epigenomics is a young field brimming with possibilities. By understanding how social interactions influence the epigenome, we can gain deeper insights into social behavior, stress responses, and even the evolution of social structures in mammals. It's a fascinating dance between genes, environment, and social interactions, rewriting the rules of the game and pushing the boundaries of our understanding of how evolution works.
Ref
Epigenomics and gene regulation in mammalian social systems





Comments
Post a Comment