Biofilms as Catalysts of Microbial Evolution: HGT, Epigenetics, and a Reconsideration of the Modern Synthesis
In a world increasingly concerned with antibiotic resistance and the rapid evolution of microorganisms, understanding the mechanisms of horizontal gene transfer (HGT) is paramount. The journal article, "Biofilms: Hot Spots of Horizontal Gene Transfer (HGT) in Aquatic Environments, with a Focus on a New HGT Mechanism," delves into the critical role of biofilms in facilitating genetic exchange among bacteria and introduces a novel mechanism for this process. The article raises intriguing challenges to the established tenets of the Modern Synthesis of evolution.
Biofilms, those ubiquitous communities of microorganisms encased in an extracellular polymeric substance (EPS) matrix, are recognized as highly conducive environments for HGT.
Their dense cellular packing, the presence of various stress factors, and the rich exchange of molecules within the EPS all contribute to a heightened frequency of gene transfer events. The article explores established HGT mechanisms such as conjugation, transformation, and transduction, detailing how these processes are amplified within the biofilm microenvironment.
Conjugation, the direct transfer of genetic material between bacterial cells via pili, is particularly efficient in biofilms due to the close proximity of cells. Transformation, the uptake of free DNA from the environment, is also enhanced as dying cells within the biofilm release their genetic material into the communal matrix. Transduction, the transfer of genes via bacteriophages, finds fertile ground in biofilms where high bacterial densities can lead to increased phage replication and subsequent gene dissemination.
The “new HGT mechanism” highlighted in the article would be a key focus. This novel mechanism could involve, for instance, membrane vesicles, which are known to carry DNA and RNA and can fuse with other cells, delivering their genetic cargo.
Alternatively, it might involve a previously uncharacterized form of DNA secretion and uptake, or perhaps a more complex interaction involving the EPS itself acting as a scaffold for gene transfer. Whatever its nature, the elucidation of this new mechanism underscores the dynamic and multifaceted strategies bacteria employ to share genetic information, thereby accelerating their adaptive potential.
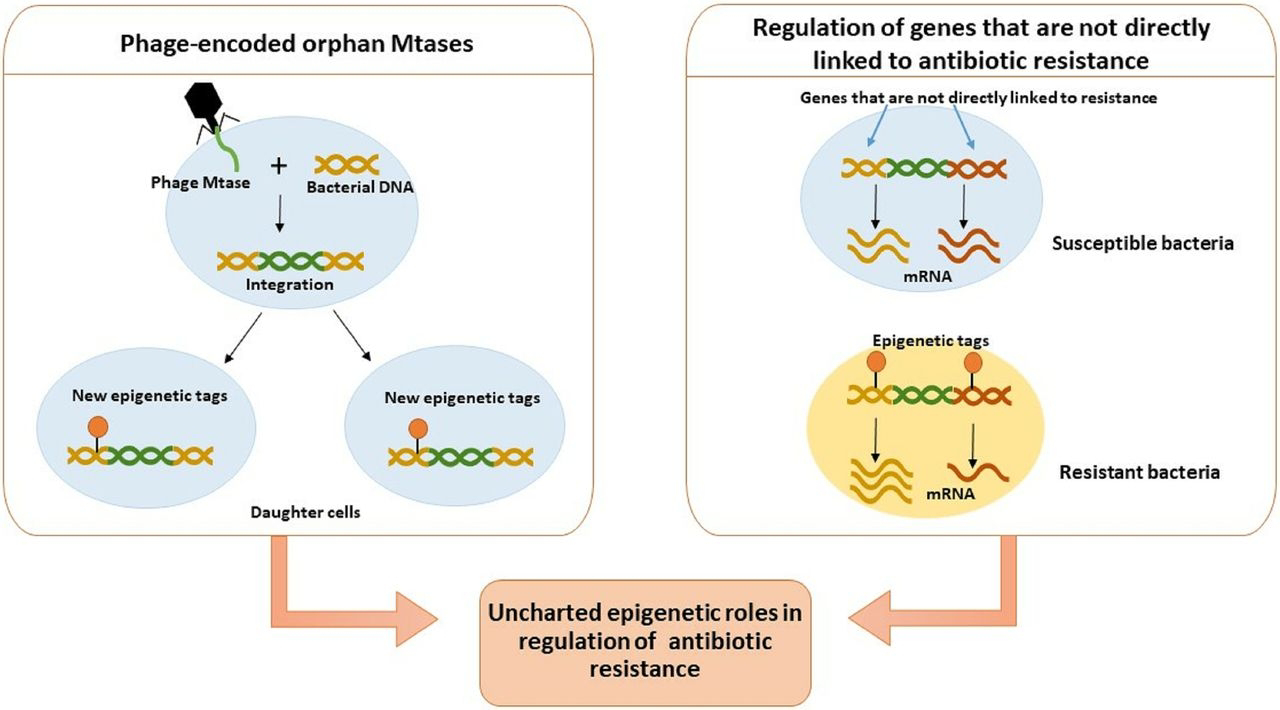
The involvement of epigenetics, focused on HGT mechanisms, can be inferred through several lenses. Epigenetics refers to heritable changes in gene expression that do not involve alterations to the underlying DNA sequence.
In bacteria, epigenetic modifications primarily involve DNA methylation and small regulatory RNAs. These modifications can influence the accessibility of DNA to transcription machinery, thereby affecting which genes are expressed and to what extent.
How does this relate to HGT in biofilms? Firstly, the uptake and integration of foreign DNA through HGT require the host cell to process and utilize this new genetic material. Epigenetic mechanisms within the recipient cell could play a crucial role in silencing or activating newly acquired genes.
For example, if a transferred gene confers antibiotic resistance, its expression might be regulated by the host's epigenetic machinery, determining the level of resistance conferred.
Conversely, if a newly acquired gene is detrimental, epigenetic silencing could act as a rapid defense mechanism, preventing its expression without permanently altering the DNA. Secondly, the very act of living within a biofilm, a stressful and competitive environment, can induce epigenetic changes in bacterial populations. These stress-induced epigenetic modifications in turn, influence the cell's propensity for HGT. For example, some epigenetic states might make a cell more "competent" for transformation, increasing its ability to take up foreign DNA. Similarly, epigenetic modifications could regulate the expression of genes involved in pilus formation for conjugation or receptors for phage attachment, thereby indirectly influencing HGT rates.The environmental cues within the biofilm (nutrient availability, presence of toxins, cell density) could trigger specific epigenetic responses that prime bacteria for genetic exchange, enabling them to rapidly adapt to fluctuating conditions.
The implications of these intertwined phenomena – HGT in biofilms and the subtle influence of epigenetics – pose a fascinating challenge to the Modern Synthesis of evolution. The Modern Synthesis, largely based on Mendelian genetics and Darwinian natural selection, emphasizes gradual evolutionary change driven by random mutations and subsequent selection of advantageous alleles.
HGT, particularly at the high rates observed in biofilms, represents a powerful mechanism for rapid, non-vertical gene acquisition, allowing bacteria to gain complex traits in a single step rather than through numerous incremental mutations. This challenges the notion of solely vertical inheritance as the primary driver of evolution.Furthermore, the involvement of epigenetics adds another layer of complexity. If environmentally induced epigenetic changes can influence HGT rates or the expression of newly acquired genes, it suggests a mechanism of rapid adaptation that is not solely reliant on DNA sequence changes. This introduces a level of phenotypic plasticity and heritability that extends beyond the traditional gene-centric view of evolution.
It suggests that environmental pressures can not only select for advantageous mutations but also induce transient, yet potentially impactful, changes in gene expression and the propensity for genetic exchange, accelerating evolutionary trajectories in ways not fully encompassed by the classical Modern Synthesis. These findings necessitate an expansion of our understanding of evolutionary mechanisms to fully account for the dynamic and rapid adaptive capabilities observed in microbial populations.





Comments
Post a Comment