The RNA Revolution: Rewriting Life's Blueprint with an Epigenetic Twist
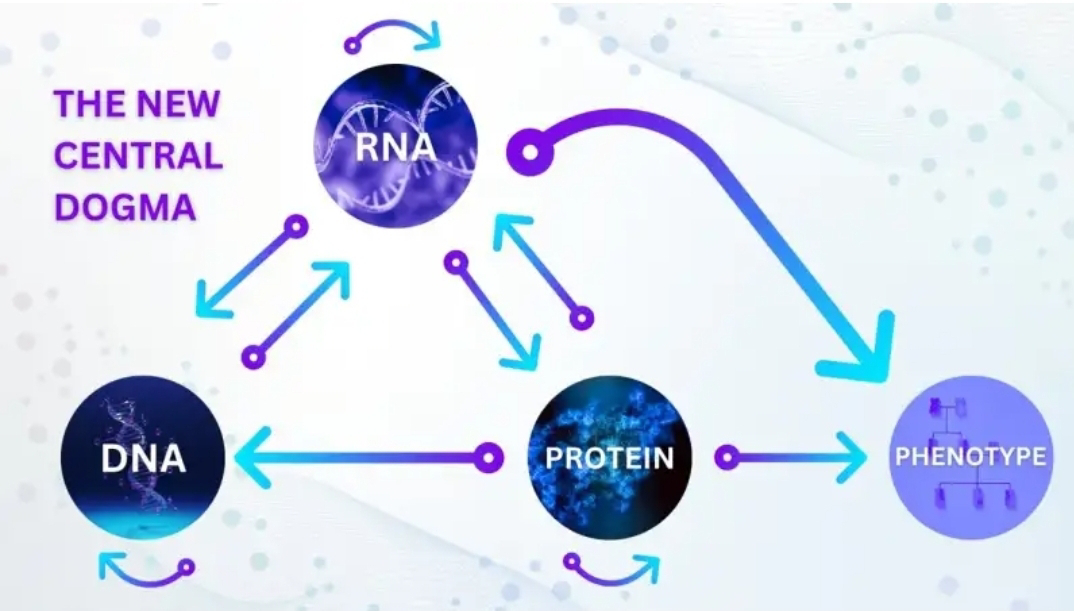
The Old Central Dogma of Evolution
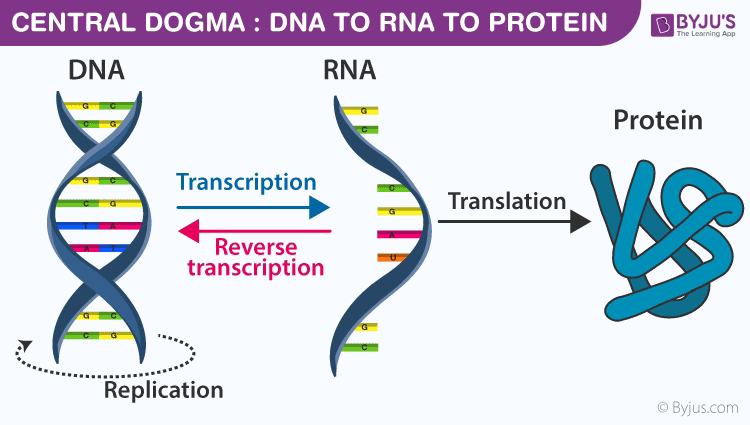
The bedrock of modern Evolution, the "central dogma," long held that DNA was the undisputed master blueprint of life, faithfully transcribed into RNA, which then dictated protein synthesis. This linear, unidirectional flow of genetic information painted a picture of a deterministic genome, where inherited traits were largely fixed by the sequence of nucleotides. However, a seismic shift is underway, a "RNA revolution" that is profoundly challenging this established paradigm and revealing a far more intricate and dynamic interplay within the cell. This revolution, meticulously detailed in the article "The RNA revolution: How our understanding of life's blueprint is being rewritten," highlights the pivotal and multifaceted roles of RNA, particularly through the lens of epigenetics, and consequently poses a significant challenge to the tenets of the Modern Synthesis of evolution.
For decades, RNA was largely relegated to the role of a mere messenger, an intermediate conduit for genetic information. The focus was squarely on messenger RNA (mRNA), the template for protein production. Yet, the past two decades have witnessed an explosion of discoveries surrounding non-coding RNAs (ncRNAs), molecules that do not translate into proteins but instead perform a bewildering array of regulatory functions. These ncRNAs, including microRNAs (miRNAs), long non-coding RNAs (lncRNAs), small interfering RNAs (siRNAs), and piwi-interacting RNAs (piRNAs), are proving to be key orchestrators of gene expression, acting at various levels from transcription to translation and beyond.
This is where epigenetics enters the fray as a central player in the RNA revolution. Epigenetics refers to heritable changes in gene expression that occur without alterations to the underlying DNA sequence. These modifications, such as DNA methylation and histone modifications, act as switches, turning genes on or off, and fine-tuning cellular responses.
Crucially, RNA molecules are not just bystanders in this epigenetic dance; they are active participants and often direct regulators. For instance, some lncRNAs have been shown to recruit chromatin-modifying enzymes to specific genomic loci, leading to localized changes in DNA methylation or histone acetylation that can silence or activate genes.
Similarly, miRNAs can repress gene expression post-transcriptionally by binding to mRNA molecules, leading to their degradation or translational inhibition, a process that can be epigenetically regulated itself.
Consider the intricate role of X-chromosome inactivation in females, a classic example of epigenetic regulation. Here, a lncRNA called Xist is instrumental in coating one of the two X chromosomes, leading to its transcriptional silencing.
This elegant mechanism ensures proper gene dosage and highlights how ncRNAs can orchestrate large-scale epigenetic remodeling of the genome. Furthermore, there is growing evidence that environmental cues can influence the expression of specific ncRNAs, which, in turn, can induce epigenetic modifications that alter gene expression patterns. This creates a fascinating feedback loop where environmental signals can translate into lasting changes in cellular function without altering the DNA sequence, a phenomenon with profound implications for development, disease, and evolution.
The escalating understanding of RNA's diverse roles, particularly its involvement in epigenetic regulation, directly challenges the core tenets of the Modern Synthesis. The Modern Synthesis, largely forged in the mid-20th century, integrated Darwinian natural selection with Mendelian genetics. It posited that evolution primarily occurs through changes in gene frequencies within populations, driven by random mutations and natural selection acting on those mutations. The genome was viewed as a relatively stable entity, with mutations providing the raw material for evolutionary change.
However, the RNA revolution and the burgeoning field of epigenetics introduce several complexities that disrupt this neat framework. Firstly, the dynamic and reversible nature of epigenetic modifications, often mediated by RNA, suggests a mechanism for rapid adaptation that does not necessitate changes in DNA sequence. If environmental stressors can induce RNA-mediated epigenetic changes that are heritable for several generations, this provides a faster, more flexible mode of response to environmental pressures than traditional random mutation and selection. Secondly, the existence of widespread ncRNA regulation implies that a significant portion of the genome, once considered "junk DNA," is functionally active and contributes to phenotypic variation. The Modern Synthesis largely focused on protein-coding genes as the primary drivers of phenotypic traits. The realization that ncRNAs regulate gene expression on a grand scale expands the scope of evolutionary mechanisms, suggesting that changes in ncRNA expression or their regulatory targets could be just as, if not more, impactful than protein-coding mutations in shaping phenotypes and driving evolutionary trajectories.
Finally, the transgenerational inheritance of epigenetic marks, even if transient, facilitated by RNA molecules, introduces a Lamarckian flavor to heredity that was largely dismissed by the Modern Synthesis. While not a wholesale endorsement of Lamarckism, the idea that acquired characteristics can be passed down, even temporarily, through epigenetic mechanisms challenges the strict separation between germline and soma and the notion that only DNA sequence changes are truly heritable.
In conclusion, the "RNA revolution" is far more than just a scientific curiosity; it is fundamentally reshaping our understanding of life's blueprint. By revealing the intricate and pervasive roles of RNA, especially its deep involvement in epigenetic regulation, the revolution is unveiling a genome that is far more dynamic, interactive, and responsive to environmental cues than previously imagined. This paradigm shift, particularly its implications for epigenetic inheritance and rapid adaptation, presents a significant and exciting challenge to the Modern Synthesis, pushing the boundaries of evolutionary biology and paving the way for a more comprehensive and nuanced understanding of how life evolves and thrives. The future of biology undoubtedly lies in unraveling the full extent of RNA's revolutionary power.



Comments
Post a Comment