Epigenetic Reprogramming: Pluripotency to Totipotency
Mammalian development begins with the fertilized zygote, a cell of remarkable potential possessing the ability to generate a full organism – a property known as totipotency.
This potential contrasts with the more restricted lineage capacity of pluripotent cells.
Totipotency is established and maintained through a complex interplay of epigenetic mechanisms, encompassing intricate changes in DNA methylation, histone modifications, chromatin remodeling, and even three-dimensional nuclear organization. This review will explore the dynamic epigenetic landscape governing the shift from pluripotency to totipotency, its implications for developmental biology, and the potential applications of this knowledge in stem cell research and regenerative medicine.
Introduction
The concept of totipotency, where a single cell holds the developmental instructions to form an entire organism, is a profound demonstration of the genome's adaptability. Understanding the mechanisms by which totipotency is established and progressively restricted into specific lineages has far-reaching implications. Epigenetic modifications, which encompass heritable changes to DNA and histones that don't alter the DNA sequence itself, are central to this process.
Epigenetic Transformations
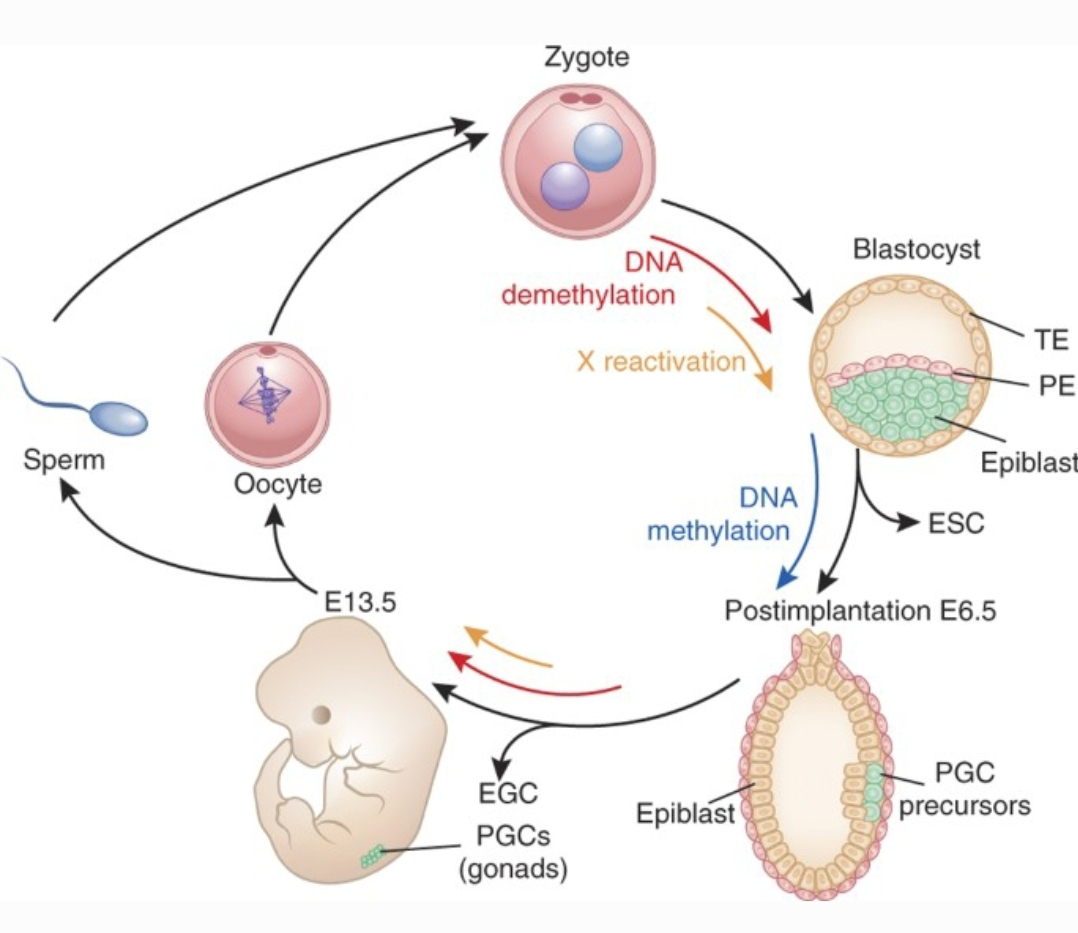
DNA Methylation Dynamics: DNA methylation plays a vital role in regulating gene expression. During the transition to totipotency, extensive demethylation erases parental epigenetic marks, followed by a wave of re-methylation to establish a new developmental blueprint. Intriguingly, certain genomic regions resist this reprogramming, suggesting the potential for transgenerational epigenetic inheritance.
Histone Modifications: A Regulatory Code: Histone modifications create a multifaceted code that dictates whether genes are accessible or inaccessible. Totipotent cells exhibit a shift towards specific histone marks associated with an open chromatin state, such as H3K4me3 and histone acetylation, fostering a permissive environment for gene expression.
Chromatin Remodeling and Nuclear Architecture: Large-scale chromatin organization and even the spatial positioning of chromosomes in the nucleus change dynamically during early development. Totipotent cells often exhibit smaller, more dispersed chromocenters, possibly linked to a state of transcriptional responsiveness. The 3D organization of the genome places distant regulatory elements, such as enhancers, in proximity to genes that influence cell fate decisions.
Non-Coding RNAs: Emerging Players: Research increasingly implicates non-coding RNAs (ncRNAs) in epigenetic regulation. Long ncRNAs are involved in processes like X-chromosome inactivation, while microRNAs contribute to post-transcriptional gene silencing. Their exact roles in totipotency are still being explored.
Zygotic Genome Activation (ZGA) and Beyond
Zygotic genome activation (ZGA) is a major milestone in achieving totipotency. ZGA encompasses a switch from reliance on maternal transcripts and proteins to expression of the zygotic genome. Specific pioneer transcription factors play critical roles in initiating ZGA by targeting closed chromatin regions and recruiting chromatin remodelers.
Implications and Applications
Developmental Biology Insights: Studies on totipotent reprogramming offer a window into how cells establish and change their identities. This information holds promise for understanding and potentially preventing developmental disorders.
Regenerative Medicine Potential: While achieving true totipotency in somatic cells remains aspirational, the insights gained from studying this transition could help refine our ability to create pluripotent cells or directly convert somatic cells into desired lineages. This unlocks potential for cell replacement therapies but also raises complex ethical considerations.
Beyond Regeneration: Totipotency and Reprogramming in Cancer: Studies hint at similarities between totipotency and the aberrant cell states found in some cancers. Understanding these parallels might provide novel therapeutic targets.
Conclusion
Epigenetic reprogramming in the journey to totipotency showcases the extraordinary versatility of the genome. Further research will undoubtedly deepen our understanding of the key factors controlling this plasticity, yield novel strategies for regenerative medicine, and illuminate the earliest moments of embryonic development.
Epigenetic Reprogramming and the Modern Synthesis: A Complex Dance
The transition from pluripotency, where cells can become any cell type within an organism, to totipotency, the unique ability of a fertilized egg to develop into an entire organism, is a remarkable feat orchestrated by complex epigenetic reprogramming. However, this process presents intriguing challenges to the established tenets of the modern synthesis, a framework uniting genetics and evolution.
The Modern Synthesis Framework
The modern synthesis, established in the mid-20th century, integrates Mendelian genetics with Darwinian evolution, explaining how genetic variation, arising from mutations and natural selection, leads to adaptations and speciation. It posits that genes, encoded within DNA, are the primary units of inheritance and evolution.
Epigenetic Reprogramming: A Wrinkle in the Framework
While the modern synthesis remains, the phenomenon of epigenetic reprogramming during the transition to totipotency introduces challenges:
Heritable Traits Beyond DNA Sequence: Epigenetic modifications, although not directly altering the DNA sequence itself, are heritable to some extent across generations. This challenges the strict focus on DNA mutations as the sole drivers of heritable traits in the modern synthesis.
Environmental Influences: External environmental factors can influence the establishment and maintenance of epigenetic patterns. This raises questions about whether and how such influences should be incorporated into the existing framework.
The Role of Epigenetics in Evolution: The potential for epigenetic modifications to influence phenotypic expression, even if indirectly, prompts inquiry into their possible role in long-term evolutionary processes, alongside genetic mutations.
Reconciling the Two Worlds: An Ongoing Debate
The field is actively debating how to integrate insights from epigenetics into the modern synthesis. Some argue for expanding the existing framework to encompass the heritable nature of epigenetic modifications and their potential for influencing phenotypes. Others propose alternative framework that explicitly account for the interplay between genetics and epigenetics in shaping biological diversity and evolution. Others propose to completely abandon the modern synthesis for an extended synthesis.
Conclusion
Epigenetic reprogramming in the transition to totipotency highlights the limitations of reducing living systems to purely genetic explanations. The modern synthesis fails to embrace the intricacies of epigenetics which is crucial for developing a more comprehensive understanding of life's complexity and the intricate dance between genes, environment, and development.
Epigenetic reprogramming in the transition from pluripotency to totipotency








Comments
Post a Comment