Horizontal Gene Transfer and its Epigenetic Implications: Challenging the Modern Synthesis
The journal article "Horizontal gene transfer potentiates adaptation by reducing selective constraints on the spread of genetic variation" presents a compelling argument for the significant role of horizontal gene transfer (HGT) in evolutionary processes, particularly in facilitating adaptation. This work highlights how HGT can effectively bypass traditional selective pressures, enabling the rapid dissemination of genetic material and, consequently, novel traits throughout a population. The mechanics of HGT and its impact on selective constraints, the intricate interplay between genetics and epigenetics is implicitly a crucial component in understanding the full ramifications of HGT-driven adaptation. Furthermore, the very premise of HGT as a potent evolutionary force fundamentally challenges tenets of the Modern Synthesis of evolution, which primarily emphasizes vertical gene transmission and gradual accumulation of mutations.
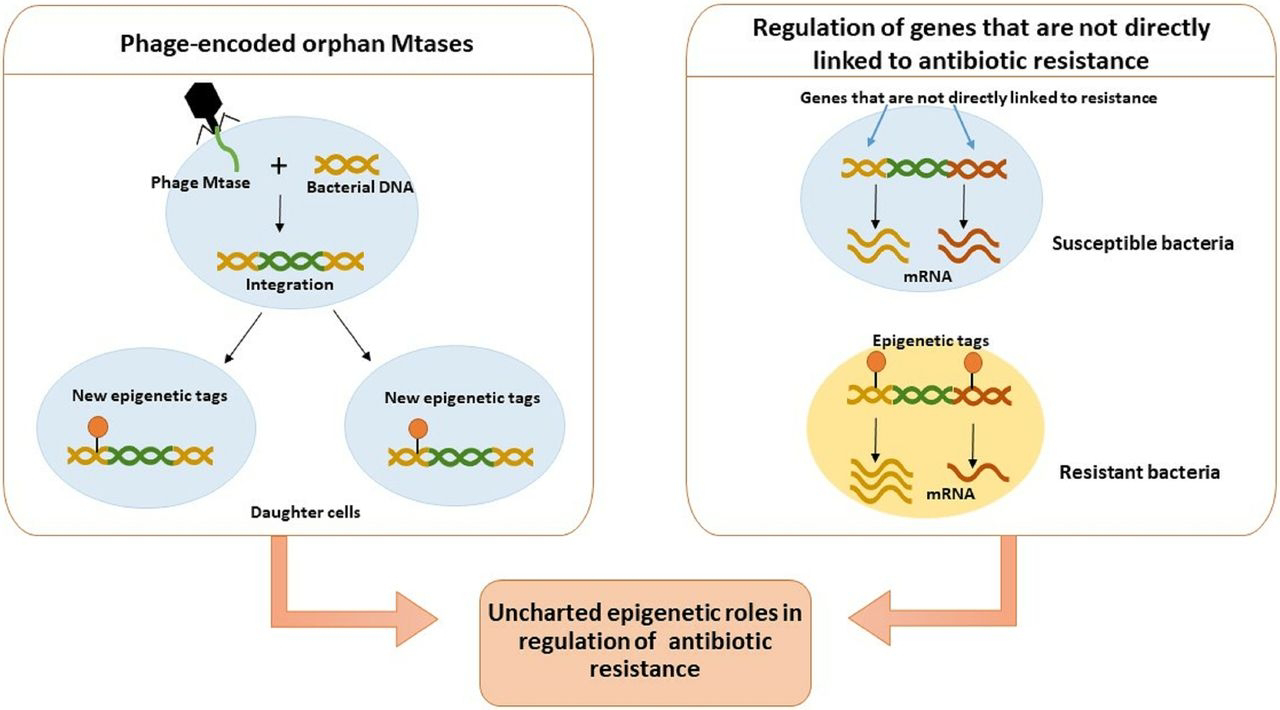
Horizontal gene transfer, the non-sexual movement of genetic material between organisms, is a well-established phenomenon, particularly prevalent in prokaryotes but increasingly recognized in eukaryotes. The core argument of the article is that HGT "potentiates adaptation by reducing selective constraints on the spread of genetic variation." In essence, when a gene is transferred horizontally, it can bypass the stringent selective filter that a vertically transmitted gene might encounter. If a beneficial mutation arises in an individual through vertical inheritance, its spread through the population is contingent on that individual's reproductive success and the advantageous nature of the trait under prevailing selective pressures. However, with HGT, a gene can be directly incorporated into the genome of a recipient organism, conferring an immediate advantage or, crucially, acting as a pre-adaptation that becomes beneficial under future environmental shifts. This mechanism allows for the rapid acquisition of complex traits, such as antibiotic resistance in bacteria or novel metabolic pathways, circumventing the lengthy process of stepwise mutation and selection.The role of epigenetics in this process is undeniably intertwined with the successful integration and expression of horizontally transferred genes. Epigenetics refers to heritable changes in gene expression that occur without alterations to the underlying DNA sequence. These changes include DNA methylation, histone modification, and non-coding RNA mechanisms, all of which influence chromatin structure and gene accessibility.When a foreign gene is introduced into a new cellular environment via HGT, its successful integration and functional expression are not solely dependent on its sequence but also on how the host cell's epigenetic machinery recognizes and processes it. For instance, a newly acquired gene might be subject to epigenetic silencing by the host's defense mechanisms, which often identify and repress foreign DNA.
Conversely, the host's epigenetic landscape might need to be reprogrammed to allow for the appropriate expression of the transferred gene, ensuring it functions harmoniously within the recipient's existing cellular networks. The article's focus on "reducing selective constraints" implicitly suggests that the epigenetic landscape of the recipient organism might be more permissive to the integration and expression of HGT-acquired genes than previously assumed, or that the rapid nature of HGT allows less time for stringent epigenetic silencing to take hold before a selective advantage is realized. The successful establishment of a horizontally transferred gene as a stable and heritable trait involves a dynamic interplay between genetic integration and epigenetic accommodation. This epigenetic flexibility or "plasticity" can thus be seen as a critical factor in potentiating adaptation via HGT, as it allows for the fine-tuning of gene expression without the need for further genetic mutations.
The implications of HGT, particularly when coupled with epigenetic considerations, pose a significant challenge to the Modern Synthesis of evolution. The Modern Synthesis, largely formulated in the mid-20th century, primarily emphasizes gradualism, the central dogma of unidirectional gene flow from DNA to RNA to protein, and the paramount importance of natural selection acting on random genetic mutations transmitted vertically from parent to offspring.
HGT directly contravenes the strict vertical transmission model, introducing a "shortcut" for genetic innovation and dissemination.
The article's assertion that HGT "reduces selective constraints" suggests a mechanism of adaptation that is far less constrained by the slow, incremental accumulation of beneficial mutations envisioned by the Modern Synthesis. If organisms can rapidly acquire entire functional gene modules from unrelated species, their evolutionary trajectories can be dramatically altered, leading to sudden, large-scale phenotypic shifts rather than purely gradual ones. This "punctuated equilibrium" via HGT offers an alternative, and potentially more rapid, pathway to adaptation in response to environmental pressures.
Furthermore, the involvement of epigenetics complicates the Modern Synthesis's emphasis on purely genetic inheritance. If epigenetic mechanisms play a crucial role in the successful integration and expression of horizontally transferred genes, then heritable changes that are not directly encoded in the DNA sequence become significant drivers of evolutionary change. This highlights a more nuanced view of inheritance, where both genetic and epigenetic information contribute to an organism's phenotype and its adaptive potential. The Modern Synthesis, while acknowledging some forms of non-genetic inheritance, largely downplayed their long-term evolutionary significance.In conclusion, the journal article sheds light on a powerful evolutionary mechanism that operates outside the traditional confines of vertical gene transmission. The role of epigenetics is undoubtedly crucial for the successful integration and expression of these transferred genes, adding another layer of complexity to the adaptive process. The very existence and demonstrated efficacy of HGT, particularly in its ability to bypass stringent selective filters and facilitate rapid adaptation, present a profound challenge to the Modern Synthesis, urging a more inclusive and dynamic understanding of how life evolves. This work underscores the need to incorporate non-traditional genetic and epigenetic mechanisms into our evolutionary frameworks, offering a richer and more comprehensive picture of the intricate processes that drive biodiversity and adaptation on Earth.






Comments
Post a Comment